Projects
Quantifying Cellular Heterogeneity
University of Washington & Institue for Systems Biology (2020-present)
Applying the concept of an epigenetic landscape for cellular differentiation to the phenotypic evolution of cancer, our aim is to gain functional insight into the non-genetic heterogeneity observed in tumors via a combination of theory and experiments. Specifically, we are developing a mathematical framework to infer phenotype transition rates using single-cell transcriptomic data from lineage tracing experiments. This project is part of an ongoing collaboration with Dr. Sui Huang at the Institute for Systems Biology in Seattle, WA, and is supported by NIH grant R01GM135396 (PI: Sui Huang).
Stochastic Thermodynamics of the Single Cell
University of Washington (2022-2023)
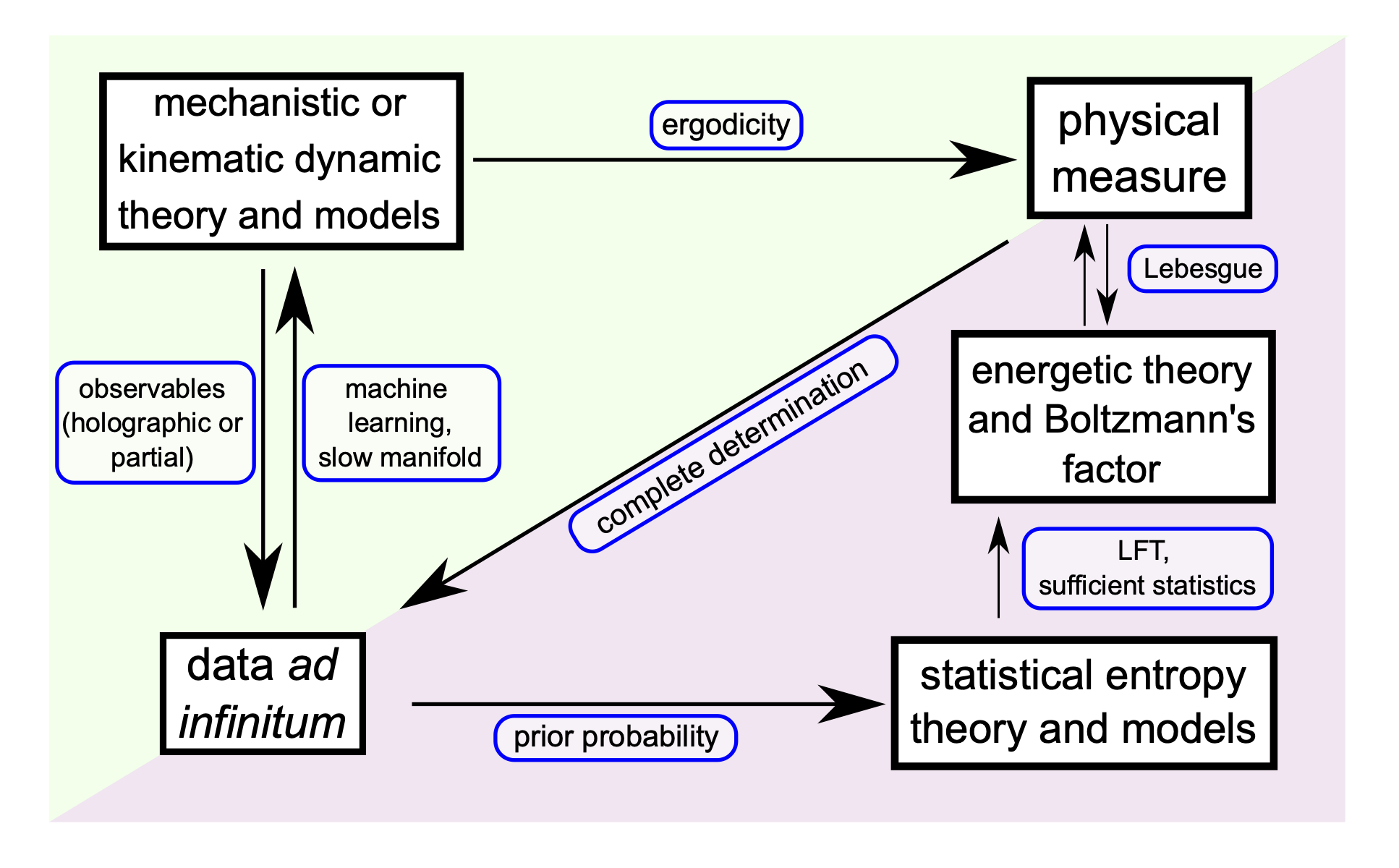
We present a treatment on statistical measurements of a collection of independent and identically distributed complex particles, showing how the concept of temperature and an ideal gas/solution law arise from this statistical analysis without relying on the concept of mechanical energy. Indeed, when sampling from an ergodic system, the data ad infinitum limit elucidates how the entropy function characterizes randomness among measurements with the emergence of a novel energetic representation for the statistics. This generalization of Gibbs' theory is applicable to statistical measurements on single living cells and other complex biological organisms, one individual at a time.
Angelini, E. and Qian, H. "Statistical analysis of random motion and energetic behavior of counting: Gibbs' theory revisited." J Phys Chem B 127(11): 2552-2564 (2023). doi: 10.1021/acs.jpcb.2c08976.
Evolutionary Dynamics of Tumor Recurrence
University of Washington & Institue for Systems Biology (2019-2022)
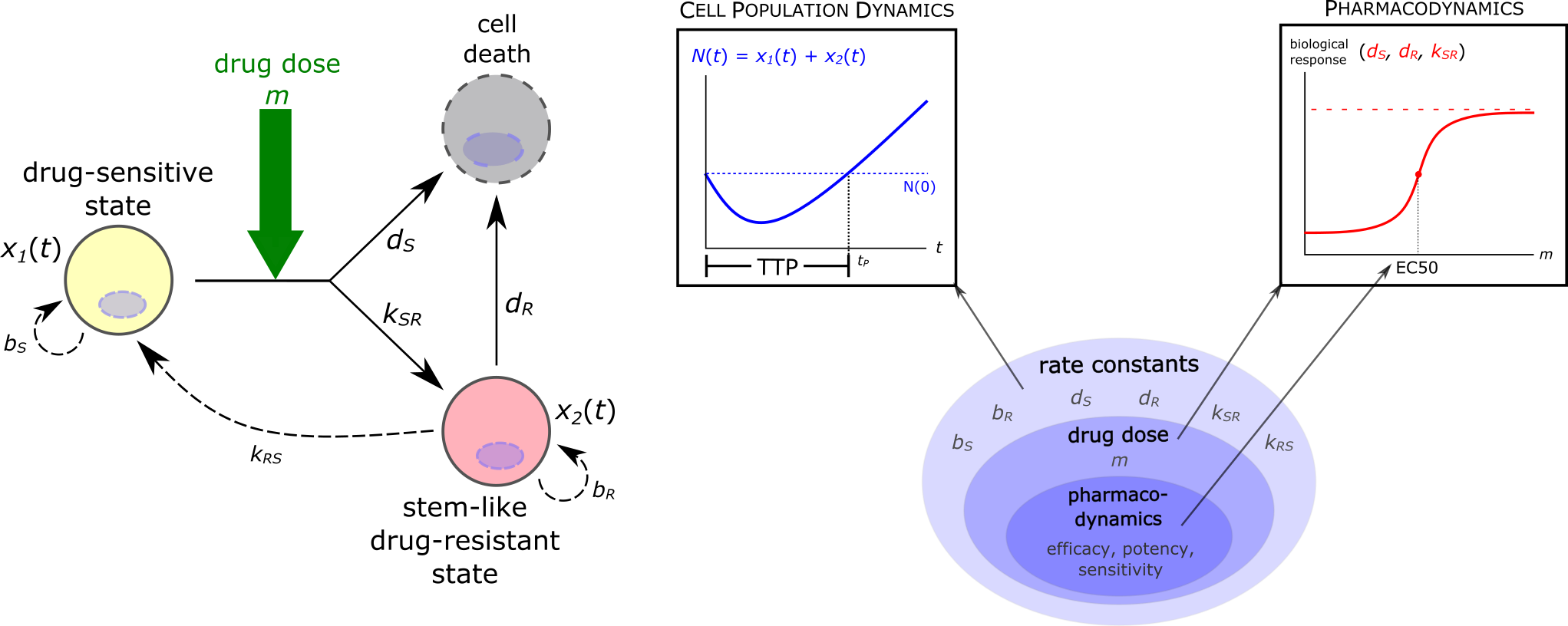
Intratumor cellular heterogeneity and non-genetic cell plasticity in tumors pose a recently recognized challenge to cancer treatment, particularly when it comes to a "stem-like" cell state that confers resistance to treatment. The double-edged-sword mechanism of treatment-induced drug resistance complements the selection of preexisting resistant cells in explaining post-treatment progression. Here, we present a generic elementary model and analytical examination of this intrinsic limitation to therapy.
Angelini et al. "A model for the intrinsic limit of cancer therapy: Duality of treatment-induced cell death and treatment-induced stemness." PLoS Comput Biol 18(7): 1010319 (2022). doi: 10.1371/journal.pcbi.1010319.